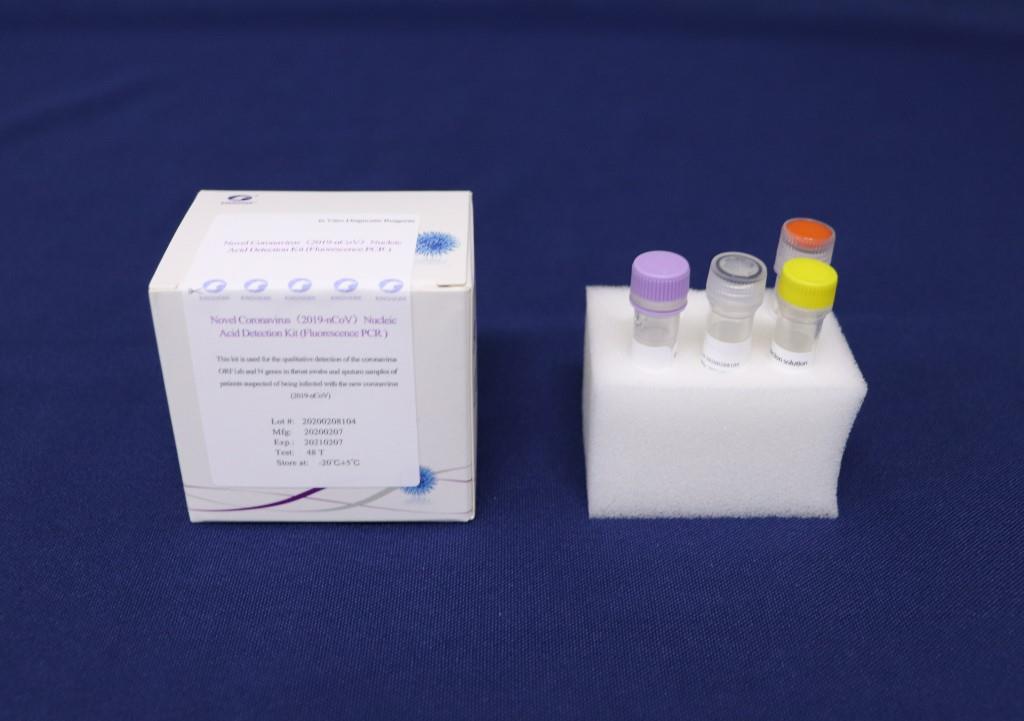
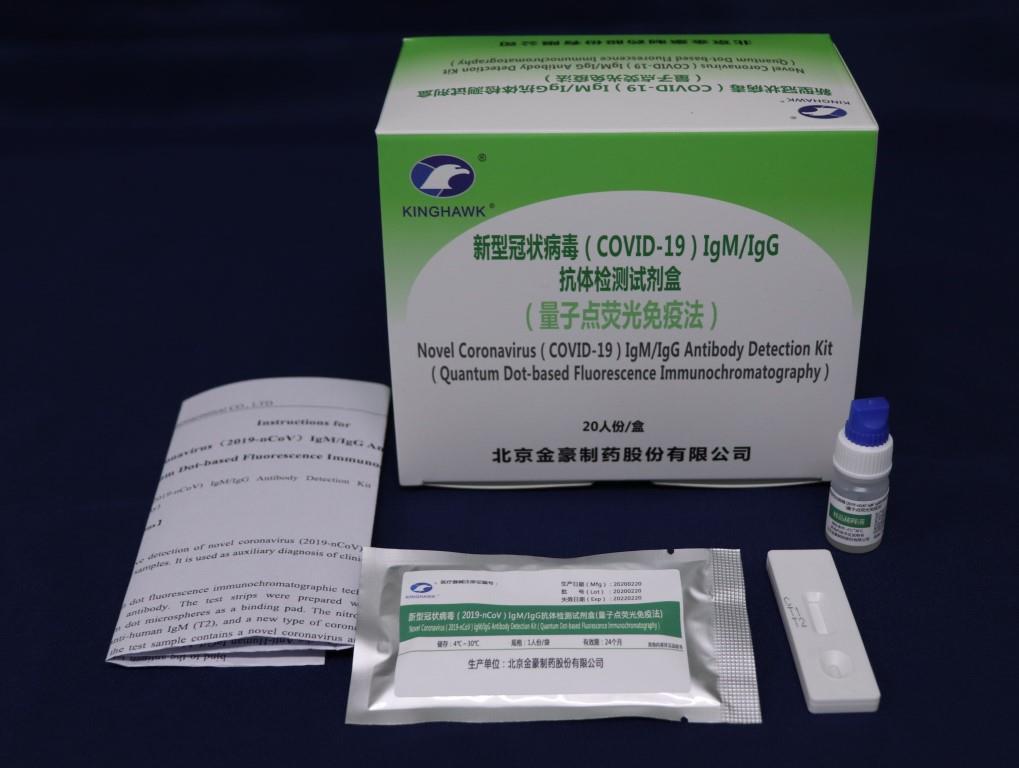
Founded in 1993 and located in Beijing Economic and Technological Development Area, Beijing Kinghawk Pharmaceutical Co.,Ltd is a hi-tech enterprise with 156 employees which covers 6000m2 and possesses 2000m2 GMP workshops, and is ISO:13485:2003, ISO:9001:2000 certified by TUV in 2006 and GMP certified by SFDA in 2003, specialized in research, development and production of in-vitro diagnostic reagents in China.
Over seventeen years of development, Beijing Kinghawk has been transformed into one of the leading IVD companies in China. Now, over 80 kinds of in-vitro diagnosis products of ELISA series, rapid detection series, clinical chemistry reagents series, antiterrorism series, PCR series and blood grouping series are manufactured and marked through out of China. Among them, the ELISA series and rapid test series are ranked the top 5 manufacturer in China, the blood grouping series contain the most completed diagnostic varieties and the antiterrorism series are the sole SFDA approved products to be used in public security area.
Besides, Kinghawk has actively undertaken several state-level research projects such as Influenza A H1N1 virus RNA (Fluorescence PCR) which was jointly developed by Kinghawk R&D Center and Chinese National Influenza Center of Chinese Center for Disease Control and Prevention, AIDS Confirmation Reagent which originated by the Health Ministry of PRC and Biological Antiterrorism Projects which originated by the Academy of Military Medical Sciences. By cooperating with foreign enterprises in Europe, North America, Japan and Australia, Kinghawk has set up extensive technical exchange channels with the new development of the Diagnostic industry and kept the same pace with global science and technology.